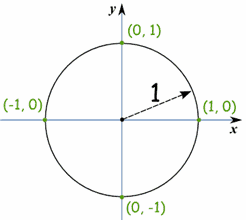
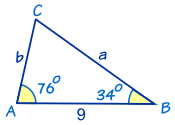
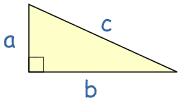
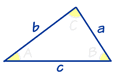
Gregor Johan Mendel was born on 20th July 1822, in the Austrian Silesia (now Hynčice, Czech Republic), his parents, Rosine and Anton were farmers and the Mendel family had lived and worked on the farm for over 130 years. Mendel was a keen gardener and Beekeeper during his childhood at the farm.
Mendel studied Physics at the University of Olomouc, and in 1843 he joined the Augustinian Abbey in Old Brno as a novice under Abbot C. F. Napp. Napp was considered to be a huge influence in his life and encouraged Mendel to continue his education. In 1851 Mendel attended the University of Vienna, where he studied physics (under Christian Doppler), Mathematics and Natural History. In 1853, Mendel returned to the Abbey to teach – primarily physics, but he also continued his gardening and from 1854-64, he carried out his famous experimental work in plant hybridization of garden peas, Pisum sativum.
In 1862, Mendel read Charles Darwin’s book The Origin of Species – at the Mendel Museum you can view the book (a German translation of the second edition) and see where Mendel made notes in the margins.
Between 1856 and 1863 Mendel cultivated and tested around 29,000 pea plants, and his study showed that one in four pea plants had purebred recessive alleles, two out of four were hybrid and one out of four were purebred dominant. His experiments led him to make two generalizations, the Law of Segregation and the Law of Independent Assortment, which later became known as Mendel’s Laws of Inheritance.
In 1865, Mendel gave lectures on his experiments at the February and March meetings for the Natural Science Society (Brno), a society Mendel himself had co-founded in 1961. In 1966 Mendel published his findings in the journalProceedings of the Natural History Society of Brünn. Unfortunately the main focus of the paper was on hybridization, not inheritance and it made little impact – being cited only a few times over the next 3 decades.
After completing his work in peas, Mendel extended his experiments to his much loved honey bees, however in 1968 Mendel was made Abbot and his experiments had to take a back seat as his time was take up with administrable duties. Mendel died on the 6th January 1884, in Brno from Chronic Nephritus. His work on inheritance was not rediscovered until the early twentieth Century, and his paper on hybridization is now considered his seminal work. Mendelian Genetics is taught to school children around the world and he is now considered to be the ‘Father’ of modern genetics.NAME: Gregor Mendel
BORN: 20th July 1822
DIED: 6th January 1884
NATIONALITY: Czech

Gregor Johan Mendel was born on 20th July 1822, in the Austrian Silesia (now Hynčice, Czech Republic), his parents, Rosine and Anton were farmers and the Mendel family had lived and worked on the farm for over 130 years. Mendel was a keen gardener and Beekeeper during his childhood at the farm.
Mendel studied Physics at the University of Olomouc, and in 1843 he joined the Augustinian Abbey in Old Brno as a novice under Abbot C. F. Napp. Napp was considered to be a huge influence in his life and encouraged Mendel to continue his education. In 1851 Mendel attended the University of Vienna, where he studied physics (under Christian Doppler), Mathematics and Natural History. In 1853, Mendel returned to the Abbey to teach – primarily physics, but he also continued his gardening and from 1854-64, he carried out his famous experimental work in plant hybridization of garden peas, Pisum sativum.
In 1862, Mendel read Charles Darwin’s book The Origin of Species – at the Mendel Museum you can view the book (a German translation of the second edition) and see where Mendel made notes in the margins.
Between 1856 and 1863 Mendel cultivated and tested around 29,000 pea plants, and his study showed that one in four pea plants had purebred recessive alleles, two out of four were hybrid and one out of four were purebred dominant. His experiments led him to make two generalizations, the Law of Segregation and the Law of Independent Assortment, which later became known as Mendel’s Laws of Inheritance.
In 1865, Mendel gave lectures on his experiments at the February and March meetings for the Natural Science Society (Brno), a society Mendel himself had co-founded in 1861. In 1866 Mendel published his findings in the journalProceedings of the Natural History Society of Brünn. Unfortunately the main focus of the paper was on hybridization, not inheritance and it made little impact – being cited only a few times over the next 3 decades.
After completing his work in peas, Mendel extended his experiments to his much loved honey bees, however in 1868 Mendel was made Abbot and his experiments had to take a back seat as his time was take up with administrable duties. Mendel died on the 6th January 1884, in Brno from Chronic Nephritus. His work on inheritance was not rediscovered until the early twentieth Century, and his paper on hybridization is now considered his seminal work. Mendelian Genetics is taught to school children around the world and he is now considered to be the ‘Father’ of modern genetics.Gregor Johan Mendel was born on 20thJuly 1822, in the Austrian Silesia (now Hynčice, Czech Republic), his parents, Rosine and Anton were farmers and the Mendel family had lived and worked on the farm for over 130 years. Mendel was a keen gardener and Beekeeper during his childhood at the farm.
Mendel studied Physics at the University of Olomouc, and in 1843 he joined the Augustinian Abbey in Old Brno as a novice under Abbot C. F. Napp. Napp was considered to be a huge influence in his life and encouraged Mendel to continue his education. In 1851 Mendel attended the University of Vienna, where he studied physics (under Christian Doppler), Mathematics and Natural History. In 1853, Mendel returned to the Abbey to teach – primarily physics, but he also continued his gardening and from 1854-64, he carried out his famous experimental work in plant hybridization of garden peas, Pisum sativum.
In 1862, Mendel read Charles Darwin’s book The Origin of Species – at the Mendel Museum you can view the book (a German translation of the second edition) and see where Mendel made notes in the margins.
Between 1856 and 1863 Mendel cultivated and tested around 29,000 pea plants, and his study showed that one in four pea plants had purebred recessive alleles, two out of four were hybrid and one out of four were purebred dominant. His experiments led him to make two generalizations, the Law of Segregation and the Law of Independent Assortment, which later became known as Mendel’s Laws of Inheritance.
In 1865, Mendel gave lectures on his experiments at the February and March meetings for the Natural Science Society (Brno), a society Mendel himself had co-founded in 1961. In 1966 Mendel published his findings in the journalProceedings of the Natural History Society of Brünn. Unfortunately the main focus of the paper was on hybridization, not inheritance and it made little impact – being cited only a few times over the next 3 decades.
After completing his work in peas, Mendel extended his experiments to his much loved honey bees, however in 1968 Mendel was made Abbot and his experiments had to take a back seat as his time was take up with administrable duties. Mendel died on the 6th January 1884, in Brno from Chronic Nephritus. His work on inheritance was not rediscovered until the early twentieth Century, and his paper on hybridization is now considered his seminal work. Mendelian Genetics is taught to school children around the world and he is now considered to be the ‘Father’ of modern genetics.Gregor Johan Mendel was born on 20thJuly 1822, in the Austrian Silesia (now Hynčice, Czech Republic), his parents, Rosine and Anton were farmers and the Mendel family had lived and worked on the farm for over 130 years. Mendel was a keen gardener and Beekeeper during his childhood at the farm.
Mendel studied Physics at the University of Olomouc, and in 1843 he joined the Augustinian Abbey in Old Brno as a novice under Abbot C. F. Napp. Napp was considered to be a huge influence in his life and encouraged Mendel to continue his education. In 1851 Mendel attended the University of Vienna, where he studied physics (under Christian Doppler), Mathematics and Natural History. In 1853, Mendel returned to the Abbey to teach – primarily physics, but he also continued his gardening and from 1854-64, he carried out his famous experimental work in plant hybridization of garden peas, Pisum sativum.
In 1862, Mendel read Charles Darwin’s book The Origin of Species – at the Mendel Museum you can view the book (a German translation of the second edition) and see where Mendel made notes in the margins.
Between 1856 and 1863 Mendel cultivated and tested around 29,000 pea plants, and his study showed that one in four pea plants had purebred recessive alleles, two out of four were hybrid and one out of four were purebred dominant. His experiments led him to make two generalizations, the Law of Segregation and the Law of Independent Assortment, which later became known as Mendel’s Laws of Inheritance.
In 1865, Mendel gave lectures on his experiments at the February and March meetings for the Natural Science Society (Brno), a society Mendel himself had co-founded in 1961. In 1966 Mendel published his findings in the journalProceedings of the Natural History Society of Brünn. Unfortunately the main focus of the paper was on hybridization, not inheritance and it made little impact – being cited only a few times over the next 3 decades.
After completing his work in peas, Mendel extended his experiments to his much loved honey bees, however in 1968 Mendel was made Abbot and his experiments had to take a back seat as his time was take up with administrable duties. Mendel died on the 6th January 1884, in Brno from Chronic Nephritus. His work on inheritance was not rediscovered until the early twentieth Century, and his paper on hybridization is now considered his seminal work. Mendelian Genetics is taught to school children around the world and he is now considered to be the ‘Father’ of modern genetics.